american academy of pediatrics covid vaccine 12-15
The Centers for Disease Control and Prevention CDC director signed off on boosters for this group Wednesday after an advisory group voted 13-1 in favor and called for a strong recommendation. Adolescents moved a step closer to being able to receive a COVID-19 vaccine after officials on Monday approved Pfizer-BioNTechs vaccine for those ages 12-15 years.

Pediatricians Want Kids To Be Part Of Covid Vaccine Trials Kaiser Health News
In a statement to the media.

. As the US. Preparing Children and Teens for Vaccination. Currently a booster shot is not recommended for children younger than 12 years old.
This study will include. Food and Drug Administration FDA authorized the Pfizer-BioNTech COVID-19 vaccine for kids ages 12 to 15 on an emergency use basis. Children and COVID Vaccine Data Report.
In the United States recent data from the American Academy of Pediatrics show that by 21 February 2022 123 million pediatric COVID-19 cases had been reported since the onset of the pandemic which represents 157 of all confirmed cases in the country 121165. US urged to fast-track vaccine approval for children under 12 as cases rise Children accounted for 15 of new Covid cases reported last week American Academy of Pediatrics data analysis shows A. Updated weekly this data report tracks the progress in vaccinating US children under 18 years of age.
The American Academy of Pediatrics AAP recommends that all eligible children and adults 5 years and older should get the COVID-19 vaccine as soon as they can. Teens ages 12 to 17 years old should receive the Pfizer-BioNTech COVID-19 booster shot at least 5 months after completing their Pfizer-BioNTech COVID-19 primary series. Continues inoculating adults and adolescents questions remain about vaccinating the 48 million kids under the age of 12.
The FDA issued an EUA for the Pfizer vaccine for kids aged 5 to 11 on 10292021 followed by ACIP recommendation and CDC approval on 1122021. The Food and Drug Administration on May 10 issued an emergency use authorization EUA for the Pfizer-BioNTech COVID-19 vaccine for the prevention of COVID-19 in individuals 12-15 years old. Which began a rapid COVID-19 inoculation campaign in December 2020 for everyone over age 15.
New vaccines are evaluated by a long-standing rigorous and transparent process through the US Food and Drug Administration and the Centers for Disease Control and Prevention CDC by which safety and efficacy data are reviewed before authorization and recommendationThe American Academy of Pediatrics AAP recommends the following. Adolescents 12-15 receive 2 doses of the adult formulation where as children. Adolescents ages 12-15 years can begin receiving COVID-19 vaccine boosters.
FDA authorizes COVID boosters for youths ages 12-15. Thats over 13 million kids who have had both of their doses of COVID-19 vaccine. The vaccine was 100 effective in preventing COVID-19 and providing strong antibody responses during clinical trials with 2260 kids ages 12 to 15 according to Pfizer.
AAP updates treatment guidance Jan. The authorization was granted after Pfizer-BioNTech announced the vaccine was found to be 100 effective in preventing COVID-19 during a clinical trial of more than 2200 adolescents ages 12 to 15. ShopAAP is the official store of the American Academy of Pediatrics.
Vaccines for children ages 5 and older may be authorized soon and clinical trials are underway in children as young as six months old. Encourage your child to keep doing their part to protect others by wearing a face mask and following other. For the latest news on COVID-19 visit httpbitlyAAPNewsCOVID19.
Ad Maximize protection get all recommended vaccine doses and boosters as soon as possible. Although the risk of children experiencing severe illness from COVID-19 is low mutations of the virus have increased infections among. The FDA first cleared the Pfizer-BioNTech vaccine through an EUA in December 2020 for those ages 16 and older.
Recommendations followed Mondays Food and Drug Administration decision to authorize emergency use of this vaccine in 12- through 15-year-old adolescents. The FDA has approved the Pfizer vaccine in teens 16 and older and granted emergency use authorization for the Pfizer vaccine for children 12 to 15. Completed enrollment of its pediatric clinical trial looking at their recombinant protein vaccine candidate against COVID-19 in adolescents 12 to 17 years old.
Download the Full Report 5042022. There were 16 COVID-19. According to a recent report by the American Academy of Pediatrics AAP.
The FDA has granted emergency use authorization for the COVID-19 vaccine to be given to children 5 to 15 years of age. 3 2022 -- The FDA also shortened the interval for boosters and authorized a third dose for certain immunocompromised children ages 5-11 years. The vaccines continue to be monitored very closely.
Full approval of the vaccine has been authorized for individuals 16 years of age and older. The American Academy of Pediatrics AAP in collaboration with the Centers for Disease Control and Prevention CDC conducted 9 key informant interviews with emerging pediatric practices to identify. Currently the Pfizer-BioNTech mRNA vaccine has been authorizedrecommended for children and adolescents aged 5-15.
Your child is considered up to date if they have received all recommended doses for their age. The AAP policy statement comes amid a discussion by the Advisory Committee on Immunization Practices of the Centers for Disease Control and. Trials showed the Pfizer-BioNTech vaccine was 100 effective and presented no serious safety concerns in adolescents leading the Food and Drug Administration FDA to grant emergency.
Over half of all kids 12 to 17 years old in the US. Were 67 000 pediatricians committed to the optimal physical mental and social health and well-being for all infants children adolescents and. 5 2022 -- Boosters can be given five months after a primary series of the Pfizer-BioNTech vaccine.
Learn more about the American Academy of Pediatrics including our mission leadership and commitment to the optimal health and well-being of all children. As COVID-19 vaccines are being administered to anyone age 12 and up Americas youngest children remain the last group that cannot get a vaccination. The North Dakota Department of Health is also recommending that all adolescents 12 17 years of age be vaccinated.
In a new policy statement the American Academy of Pediatrics AAP recommends vaccinating all children ages 12 and older who are eligible for the federally authorized COVID-19 vaccine. The Food and Drug Administration FDA authorized COVID-19 vaccine boosters for adolescents ages 12-15 years on Monday amid surging case counts. The best way to slow the emergence of COVID variants is to get vaccinated.
Almost one-fourth of all kids 5 through 11 have had at least one dose. All purchases directly benefit and support the health and well-being of all infants children adolescents and young adults. Health officials also shortened the time interval for boosters after a Pfizer-BioNTech primary series and will allow a third primary series dose for certain immunocompromised children ages 5-11 years.
On May 12 2021 CDC approved the use of the Pfizer-BioNTech COVID-19 vaccine in persons aged 12-15 years following the vaccines EUA granted by the FDA on May 10th. Have been fully vaccinated.
Covid 19 Vaccine For Children And Adolescents Canadian Paediatric Society
Aap Advises Children And Teens Age 12 And Up Get The Covid 19 Vaccine Healthychildren Org
Back To School White House Pushes For Kids 12 And Up To Get Covid Vaccine
Covid Vaccines For Kids Under 12 Expected Midwinter Fda Official Says

Covid 19 Vaccine For 12 To 15 Year Olds What Parents Should Know Methodist Health System Omaha Council Bluffs Fremont

Faq Covid 19 Vaccine For Children Phoenix Children S Hospital

Aap Recommends Covid 19 Vaccine For Ages 16 And Up
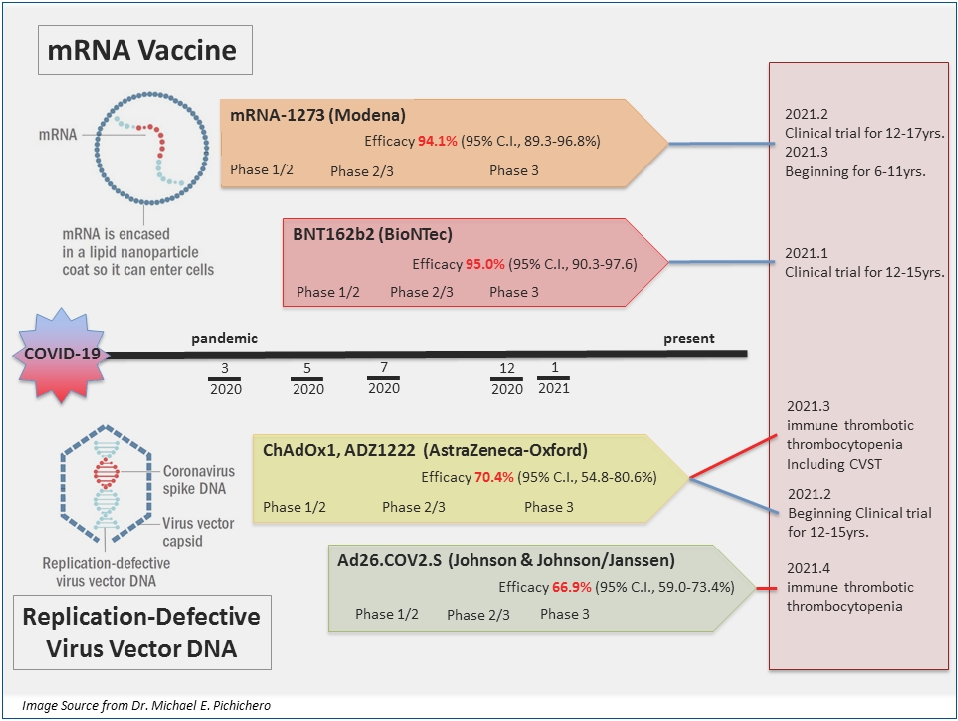
Updates On The Coronavirus Disease 2019 Vaccine And Consideration In Children

Covid 19 Vaccine For Kids Benefits Are Many Here Are Answers To Parental Concerns Ask The Pediatrician Column Together Lancasteronline Com

As School Resumes Uf Health Pediatrics Expert Gives Advice On Covid 19 Safety Uf Health University Of Florida Health
Kids Covid Vaccine Side Effects What To Know
Fda Authorizes Pfizer Covid Vaccine For Children 12 To 15

Kids Covid Vaccine Twitter Chat Twitter
Will Vaccinating Kids Under 12 Get The U S To Herd Immunity

Fact Check How Useful Are Coronavirus Vaccines For Children And Adolescents Coronavirus And Covid 19 Latest News About Covid 19 Dw 06 08 2021

Covid Vaccines In Teens And Heart Inflammation What You Need To Know Shots Health News Npr

Cdc Committee Recommends That 12 To 15 Year Olds Receive The Pfizer Biontech Covid Vaccine
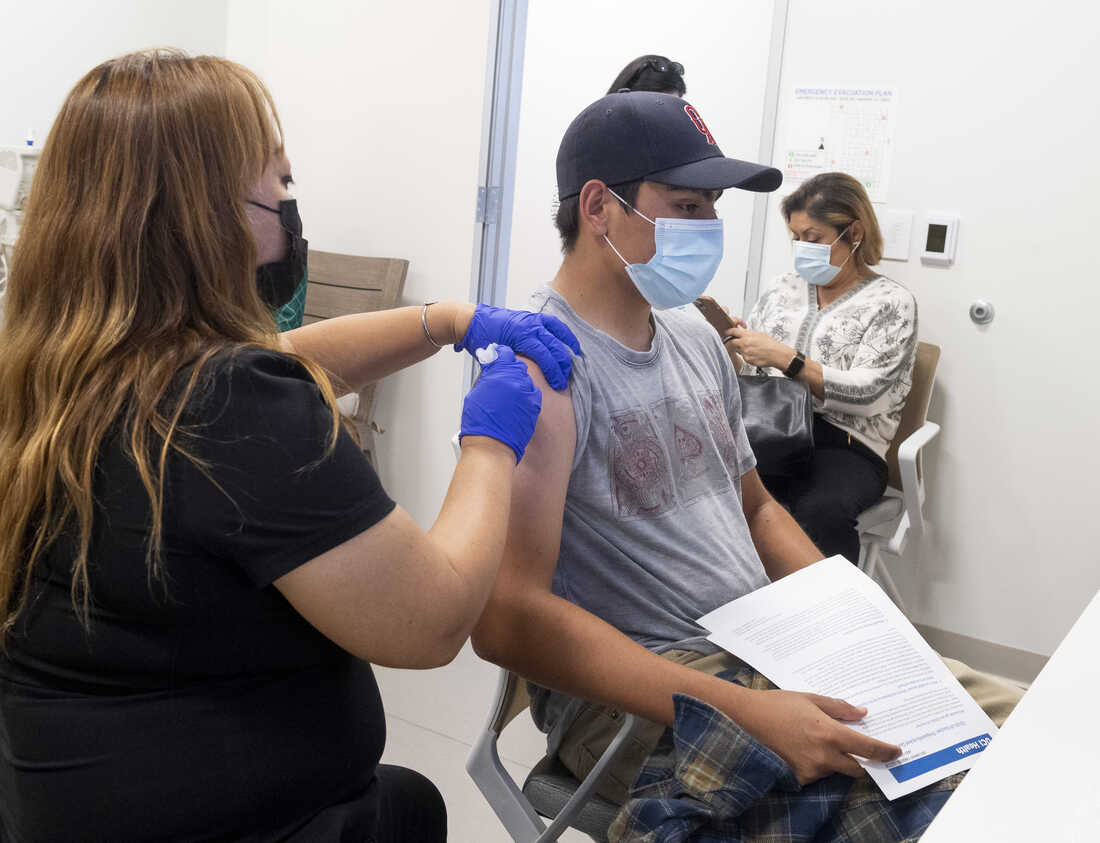
Faq About Pfizer S Covid Vaccine And Kids Shots Health News Npr

Cdc Panel Recommends Pfizer Biontech Vaccine For Kids 12 To 15 Years Old